5 Six methods of preparing Ni(OH) 2. (a) Basification of a nickel(II)... | Download Scientific Diagram

Activity Origins and Design Principles of Nickel-Based Catalysts for Nucleophile Electrooxidation - ScienceDirect

Ultrathin Nickel Hydroxide and Oxide Nanosheets: Synthesis, Characterizations and Excellent Supercapacitor Performances | Scientific Reports

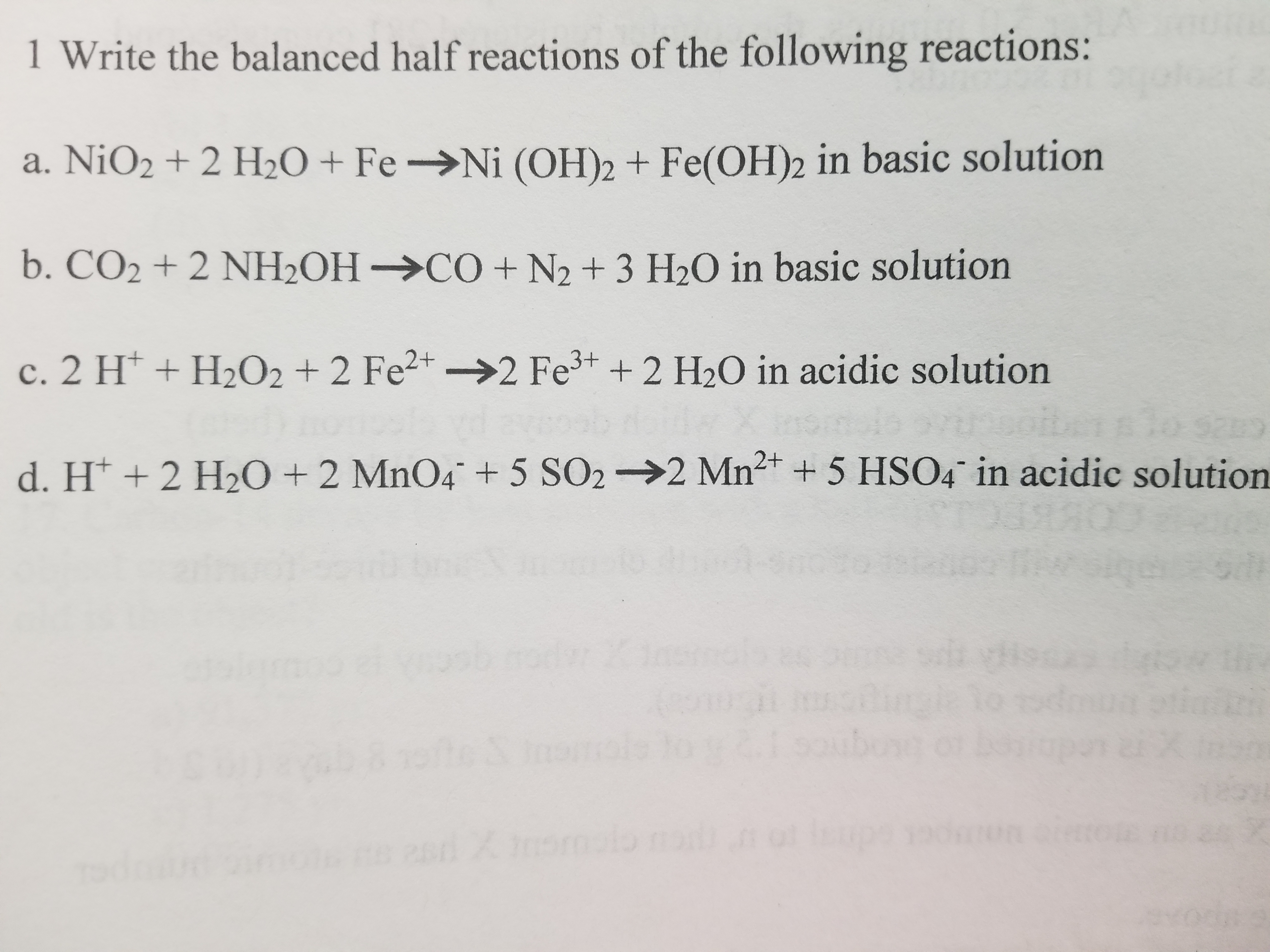
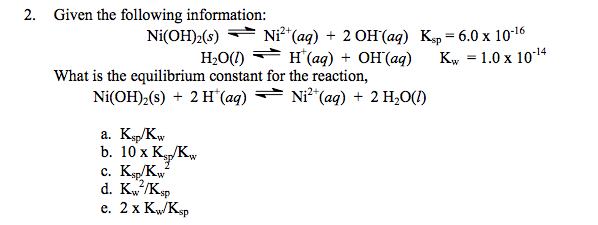
SOLVED: The solubility of solid nickel hydroxide, Ni(OH)2, is governed by its Ksp= 6x10^-16. a) Write the equation for this dissolution reaction, and the equilibrium expression. b) Nickel ions undergo three complexations

Metallic Gold-Incorporated Ni(OH)2 for Enhanced Water Oxidation in an Alkaline Medium: A Simple Wet-Chemical Approach | Inorganic Chemistry

Nanoflower Ni(OH) 2 grown in situ on Ni foam for high-performance supercapacitor electrode materials - Sustainable Energy & Fuels (RSC Publishing) DOI:10.1039/D1SE01036K

Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors | ACS Applied Materials & Interfaces

Metallic Gold-Incorporated Ni(OH)2 for Enhanced Water Oxidation in an Alkaline Medium: A Simple Wet-Chemical Approach | Inorganic Chemistry

NiSe2/Ni(OH)2 Heterojunction Composite through Epitaxial-like Strategy as High-Rate Battery-Type Electrode Material | SpringerLink
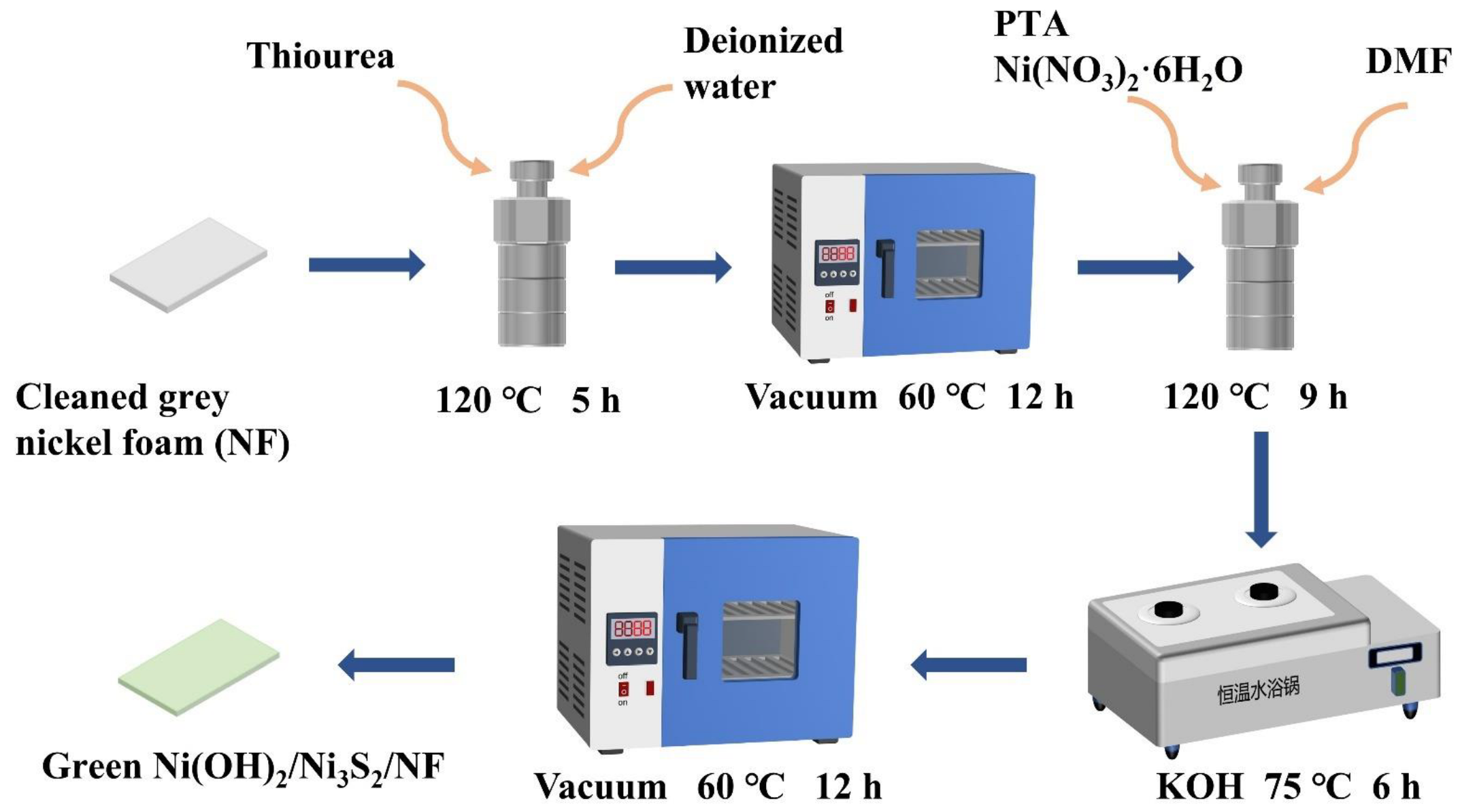
Nanomaterials | Free Full-Text | Synthesis of Ni3S2 and MOF-Derived Ni(OH)2 Composite Electrode Materials on Ni Foam for High-Performance Supercapacitors

Hydrolysis of Methoxylated Nickel Hydroxide Leading to Single-Layer Ni(OH)2 Nanosheets | Inorganic Chemistry

Metallic Gold-Incorporated Ni(OH)2 for Enhanced Water Oxidation in an Alkaline Medium: A Simple Wet-Chemical Approach | Inorganic Chemistry

Nickel hydroxides and related materials: a review of their structures, synthesis and properties | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences

Speciation diagram of Ni 2+ species as a function of solution pH. The... | Download Scientific Diagram

Visible-Light Photocatalytic H2 Production Activity of β-Ni(OH)2-Modified CdS Mesoporous Nanoheterojunction Networks | ACS Catalysis

Effects of Fe Electrolyte Impurities on Ni(OH)2/NiOOH Structure and Oxygen Evolution Activity | The Journal of Physical Chemistry C